Hyperkalaemia
Disclaimer
These guidelines have been produced to guide clinical decision making for the medical, nursing and allied health staff of Perth Children’s Hospital. They are not strict protocols, and they do not replace the judgement of a senior clinician. Clinical common-sense should be applied at all times. These clinical guidelines should never be relied on as a substitute for proper assessment with respect to the particular circumstances of each case and the needs of each patient. Clinicians should also consider the local skill level available and their local area policies before following any guideline.
Read the full CAHS clinical disclaimer
|
Aim
To guide Emergency Department (ED) staff with the assessment and management of acute hyperkalaemia.
Definition
Hyperkalaemia is defined as a serum potassium of more than 5.5 mmol/L.
Background1,2
- Serum potassium over 6.5 – 7 mmol/L, especially when associated with electrocardiogram (ECG) changes is potentially life-threatening, and should be treated as an emergency.
- Cardiac toxicity is enhanced by hypocalcaemia, hyponatraemia or acidosis, and patients with these abnormalities may experience complications at lower potassium levels.
Key points
In children, severe hyperkalaemia may result from:
- drug ingestions (e.g. digoxin, angiotensin-converting enzyme (ACE)-inhibitors, oral potassium)
- acute renal failure
- massive tissue damage (major trauma or burns, tumour lysis syndrome, haemolysis)
- severe metabolic acidosis
- adrenogenital syndromes.
Assessment
- Perform an ECG
- Exclude erroneous high potassium (pseudo-hyperkalaemia) due to haemolysis during collection or transport of the specimen.
History
Clinical features of hyperkalaemia relate to potassium’s effect on cellular membrane polarisation.
- Early symptoms include nausea, vomiting and paraesthesia.
Examination
Assess for:
- muscle weakness, progressing to flaccid paralysis and respiratory failure.
- cardiac conduction disturbance, resulting in wide complex tachycardia, ventricular fibrillation and circulatory failure.
Investigations
ECG changes2
- In acute hyperkalaemia, cardiac conduction disturbance results in ECG changes which correlate roughly with serum potassium levels.
| Serum potassium
> 6 mmol/L
|

|
- Tall, symmetrical peaked T-waves
|
|
Serum potassium > 7.5 mmol/L
|

|
- PR interval lengthens (1st degree atrioventricular (AV) block)
- Widened QRS (intraventricular block)
|
|
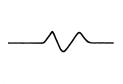
Serum potassium > 9 mmol/L
|
|
- Absent P-wave
- Pre-arrest, QRS and T-waves merge to form a sine wave
|
Management
- Hyperkalaemia should be treated when serum potassium is over 7 mmol/L, or at levels lower than this if ECG changes are present.
- Emergency management of hyperkalaemia should include early consultation with the Paediatric Critical Care (PCC).
Step 1: Protect the myocardium from the effects of hyperkalaemia
- Discontinue any potassium supplement and potassium-containing IV fluids.
|
Calcium
Refer to Calcium Monograph - Medication Management Manual (internal WA Health only)
Refer to Calcium Gluconate 10% Monograph – Neonatal Medication Monographs (internal WA Health only)
|
- 10% calcium gluconate 0.5mL/kg (maximum 20mL) IV over 2-5 minutes.2,3
(10% calcium gluconate = 2.2mmol calcium in 10mL)3
OR
- 10% calcium chloride 0.2mL/kg (maximum 10mL) IV over 2-5 minutes3,4
(10% calcium chloride = 6.8mmol calcium in 10mL)3
|
- Doesn't lower the serum potassium but is cardio-protective in that it stabilises the myocardium, reducing the risk of arrhythmias.
- Contraindicated in digitalis toxicity and hypercalcaemia.
- Dose can be repeated after 10 minutes if ECG is still abnormal1,2.
- Time to onset of action is immediate1,2.
- The effects only last for 30–60 min1,2
- Can be given as either calcium gluconate or calcium chloride.
The calcium content of each is different – take care with dosing.
|
Step 2: Lower the serum potassium level urgently
- Note: All of these methods act by shifting potassium intracellularly, thereby reducing the serum potassium level. None of these methods actually reduce total body potassium.
- These methods are not listed in specific treatment order – the clinical situation and clinician decision will guide choice of management.
|
Salbutamol2
Refer to Salbutamol - Neonatal Medication Protocols
Refer to Salbutamol – Medication Management Manual
|
Nebulised:
1 month to 18 years:
- <25kg: 2.5mg
- ≥25kg: 5mg
- Repeat if necessary1,2
Onset of action: 20-30min
Salbutamol should not be used as the sole agent for treating moderate to severe hyperkalaemia.
IV:
Use for severe hyperkalaemia – discuss with senior doctor before administering:
- 1 month to 18 years: 4microg/kg (max 250microg). Repeat if necessary1.
Administration: Suitable for the 500 microg/mL ampoule only- dilute dose to 50microg/mL and administer over 5 minutes3.
|
- Nebulised or intravenous.
- As effective as glucose and insulin.
- Acts within 60 minutes and lasts about 6 hours.
|
|
Glucose
|
- Glucose 10% at 5mL/kg as a slow IV bolus at 5–10mL/kg/hr1,2
Then commence:
- glucose 5% + sodium chloride 0.9% at maintenance rate
|
- Give glucose prior to or with insulin infusion.
- Similar onset and duration of effect to salbutamol.
- A patient’s endogenous insulin drives potassium intracellularly.
- Monitor blood glucose every 30 minutes.
|
|
Insulin
Refer to Insulin Monograph – Medication Management Manual (internal WA Health only)
Refer to Neonatal Medication Protocols (internal WA Health only)
|
- Insulin short acting neutral (Actrapid ®) infusion at 0.05-0.1 unit/kg/hr1
- Make up 50 units of Actrapid® in 50mL sodium chloride 0.9% (1unit/mL)3.
- Prime line with 20mL of solution before commencing the infusion.
|
- Discuss with a senior doctor before commencing.
- Only to be used with IV glucose if hyperglycaemia is an issue (blood glucose >10mmol/L)1
- Insulin is only to be given once the glucose infusion has been commenced.
- Onset of action begins within 30-60 min and can last for up to 8 hours.1
- Monitor glucose every 30-60 minutes.
|
|
Sodium bicarbonate
|
- Infuse at 1 mmol/kg intravenous over 10-15 minutes.1,2
|
- Discuss with an Emergency Department senior doctor or PCC prior to use.
- Not routine but can be used in an emergency even in the absence of metabolic acidosis.
- Do not administer via the same line as calcium.
- Contraindicated in alkalosis, hypernatraemia.
- Any hypocalcaemia must first be corrected.
- Onset of action - 15 minutes, effects can last for up to 2 hours1,2
|
|
Furosemide2
(Frusemide)
Refer to Furosemide (Medication Management Manual
Refer to Neonatal Medication Protocols
|
- 1mg/kg (IV) administered over 5 minutes.1,2
|
- Consider (in patients in fluid overload in consultation with PCC)
- The onset of effect is 1 to 2 hours.
- May be repeated after 6 hours
- Be wary of hypovolaemia
|
Step 3: Promote elimination of potassium from the body
|
Polystyrene sulfonate resins (available in calcium and sodium forms)
Sodium polystyrene sulfonate1
(Resonium-A)
or
Calcium polystyrene sulfonate5
(Calcium Resonium)
|
Oral/rectal dose
- 0.5 - 1g/kg (max 30g daily)5.
Oral administration
- mix with 3–4 mL of water or syrup per gram of resin (do not use fruit juice)5.
- Onset: 1-2 hours6
Dose may be repeated in 6-8 hours based upon serum potassium6
Rectal administration
- Refer to AMH CDC for administration advice.
|
- Cease polystyrene sulfonate resin treatment when the serum potassium is less than 5 mmol/L (to avoid hypokalaemia)5
- Do not use if the patient has ileus, has had recent abdominal surgery or perforation.
- Do not use sodium polystyrene sulfonate if the patient is hypernatraemic.7
* Consider the use of calcium polystyrene sulfonate in these patients.
- Separate oral administration of the resin from other oral medications by at least 3 hours.5
|
|
Dialysis
|
- Peritoneal dialysis or haemodialysis
|
- Discuss with PCC / renal team
- Peritoneal dialysis or haemodialysis.
|
Nursing care
- Continuous ECG monitoring is required due to the risk of lethal dysrhythmias.
- Minimum full set of hourly observations recorded on the Observation and Response Tool with additional information on the Clinical Comments chart.
- Monitor blood glucose levels as required by treatment regime.
- Maintain accurate fluid balance record and monitoring including baseline patient weight.
References
- Masilamani K, van der Voort J. The management of acute hyperkalaemia in neonates and children. Arch Dis Child 2012; 97:376.
- Somers MJ, Management of hyperkalemia in children - Pediatric hyperkalemia management rapid review. UpToDate Last updated: 2 June 2022 Cited: 6 April 2022 Available from: Management of hyperkalemia in children - UpToDate (health.wa.gov.au)
- Symonds K & Ermer, J editors. Australian Injectable Drugs Handbook. Eighth edition. Collingwood, Vic: The Society of Hospital Pharmacists of Australia; 2022, [available from: AIDH - CALCIUM GLUCONATE (health.wa.gov.au)]
- Calcium chloride. Clinical Pharmacology powered by ClinicalKey. Philadelphia (PA): Elsevier, 2022. Available from: http://www.clinicalkey.com.pklibresources.health.wa.gov.au
- AMH Children’s Dosing Companion (2021) Australian Medicines Handbook Pty Ltd 2021, [Internet] Polystyrene sulphonate resins; [Modified July 2021, Cited 10 February 2022] Available from: Polystyrene sulfonate resins - AMH Children's Dosing Companion (health.wa.gov.au)
- Chaitman M, Dixit D, Bridgeman MB. Potassium-Binding Agents for the Clinical Management of Hyperkalemia. Pharmacy & Therapeutics. 2016;41(1):43-50.
- Filippi L, Cecchi A, Dani C, et al. Hypernatraemia induced by sodium polystyrene sulphonate (Kayexalate) in two extremely low birth weight newborns. Paediatr Anaesth 2004;14:271–5.
| Reviewer/Team: |
ED HOD, ED Consultants, ED CNM, ED CNS, Pharmacist
|
Last reviewed: |
May 2022 |
|
|
Review date: |
Mar 2025 |
This document can be made available in alternative formats on request for a person with a disability.