Clinical care of paediatric patients with COVID-19
Disclaimer
These guidelines have been produced to guide clinical decision making for the medical, nursing and allied health staff of Perth Children’s Hospital. They are not strict protocols, and they do not replace the judgement of a senior clinician. Clinical common-sense should be applied at all times. These clinical guidelines should never be relied on as a substitute for proper assessment with respect to the particular circumstances of each case and the needs of each patient. Clinicians should also consider the local skill level available and their local area policies before following any guideline.
Read the full CAHS Clinical disclaimer
|
Aim
To provide guidance on the clinical care of paediatric patients diagnosed with SARS-CoV-2 infection and those identified as a close contact.
Key points
- These guidelines are based on current available knowledge and will change as more evidence becomes available about SARS-CoV2 infection and the disease it causes, COVID-19.
- Most children with COVID-19 have a mild illness which may be indistinguishable from other common endemic viral illnesses (e.g. respiratory syncytial virus (RSV), influenza A/B).
- Pre-existing treatment protocols and guidelines for common paediatric conditions such as bronchiolitis, croup, viral wheeze/asthma and lobar pneumonia should be followed in addition those covering the management of COVID-19.
- All efforts should be made to reduce the need for aerosol generating procedures (AGPs), and especially so in COVID-19 positive patients and close contacts. If an AGP is required, HCWs should use a fit tested particulate filtration respirator (PFR) and protective eyewear.
- In alignment with the WA Health policy Coronavirus Disease - 2019: Infection Prevention and Control in Western Australian Healthcare Facilities, it is advised that all COVID-19 positive patients admitted to hospital are admitted to a single room, preferably a negative pressure isolation room (NPIR) with anteroom. If unavailable, a single room with door closed and HEPA filter in place is a reasonable alternative. Personal Protective Equipment (PPE) consistent with Standard, Contact & Airborne precautions (gown, gloves, P2/N95 respirator and protective eyewear) should be used when entering the room of a COVID-19 positive patient or close contact.
Case definitions
Refer to the COVID-19 Communicable Diseases Network of Australia (CDNA) Guidelines for Public Health Units for up to date information & case definitions.
Patients who meet the following confirmed and probable case definitions should be managed as a positive COVID-19 case:
Confirmed case
The confirmed case definition intends to capture newly diagnosed cases with laboratory definitive evidence to support a diagnosis.
Laboratory definitive evidence:
- Detection of SARS-CoV-2 by nucleic amplification acid testing (NAAT); or
- Isolation of SARS-CoV-2 in cell culture, with confirmation using a NAAT; or
- SARS-CoV-2 IgG seroconversion or a four-fold or greater increase in SARS-CoV-2 antibodies of any immunoglobulin subclass including ‘total’ assays in acute and convalescent sera, in the absence of vaccination
Probable case
A probable case includes individuals who have laboratory suggestive evidence.
Laboratory suggestive evidence:
- Detection of SARS-CoV-2 by rapid antigen testing (RAT)
Close contact
A COVID-19 close contact is defined as:
Reinfection
A subsequent confirmed or probable SARS-CoV-2 infection in a person with a recent history of confirmed or probable COVID-19 that is determined to be separate to the first infection based on clinical, epidemiological &/or laboratory findings.
Who to test for SARS-CoV-2?
- SARS-CoV-2 NAAT remains the gold standard for diagnosing acute symptomatic SARS-CoV-2 infection in the hospital setting and should be requested where system capacity allows.
- SARS-CoV-2 RAT offers a suitable alternative to diagnosis
- All people with COVID-19 compatible symptoms as listed below should continue to be tested for SARS-CoV-2:
- Acute respiratory symptoms: cough, sore throat, rhinorrhoea/nasal congestion and/or shortness of breath and/or
- Fever ≥37.5°C where no other cause is identified [Patients who have an alternate cause for their fever identified (e.g. urinary tract infection, appendicitis) do not meet this diagnostic criterion] and/or
- Other non-specific symptoms: headache, myalgia, fatigue, nausea, vomiting, diarrhoea, loss of appetite or loss of taste or smell (less common with new variants).
- All patients with clinical features of COVID-19 infection, as outlined above, should be tested for SARS-CoV-2 using a NAAT if being admitted to hospital. For PCH, the Biofire respiratory panel is recommended. It includes SARS-CoV-2 in addition to several other common viral and bacterial respiratory pathogens.
- In the community setting following a positive test for SARS-CoV-2, further testing is not recommended during the associated isolation period and is not required for the purpose of release from isolation.
- In the hospital inpatient setting, decisions to de-isolate a patient, and the necessity for repeat COVID-19 RAT, should be discussed with the Infection Prevention & Control team.
Testing after COVID-19 infection
Natural infection with SARS-CoV-2 provides some protection against reinfection, although reinfection does occur.
- All patients with COVID-19 compatible symptoms 4 or more weeks after being a COVID-19 case should be tested for SARS-CoV-2.
- Patients who develop new symptoms within 4 weeks of an acute COVID-19 infection should be tested for respiratory viruses, including SARS-CoV-2, in situations where a diagnosis will inform clinical or public health management or where hospital admission is required.
Asymptomatic patients
- As we progress through the pandemic, testing recommendations have changed with testing now primarily targeted at symptomatic individuals.
- Asymptomatic screening/surveillance with SARS-CoV-2 RAT in the healthcare setting is now largely confined to:
- Cohorts of patients with a higher risk of severe disease
- Identified COVID-19 close contacts
How to test
Nasopharyngeal specimen collection and transport for COVID-19 NAAT (PCR):
- Clinicians must complete a pathology request form & should include patient symptoms & presence of COVID-19 risk factors (i.e. household close contact)
- For patients admitted to PCH through the Emergency Department or on the wards, a rapid respiratory virus PCR panel (“Biofire respiratory multiplex PCR”) should be requested
- Note that patients cannot be sent to the PCH PathWest Collection Centre for the purpose of COVID-19 swab collection as patient flow requirements for COVID-19 testing clinics cannot be maintained at this site
- Use a single dry nasopharyngeal flocked swab for PCR testing:
- Measure the distance from the nose to the ear to provide an estimate of the distance to insert the swab
- The swab should be inserted once into the nasopharynx by directing the swab in line with the nasal floor. This is a specific CAHS recommendation as multiple site sampling is potentially distressing for children without clear evidence of increased viral detection
- Once the swab has been inserted, leave for a few seconds to absorb secretions and then rotate on withdrawal
- Re-sheath swab following collection
- Do NOT pour transport medium into the sheath as they are not designed to hold liquid
- Place the specimen into a plastic specimen bag for transport to PathWest at QEII. Swab samples collected for COVID-19 PCR can be sent to the central specimen reception at PathWest QEII via the pneumatic tube system
- Urgent samples should be walked to PathWest Central Specimen Reception, Ground floor PP Block. No additional PPE is required for those delivering swabs
Figure 1: Paediatric flocked swab and sheath
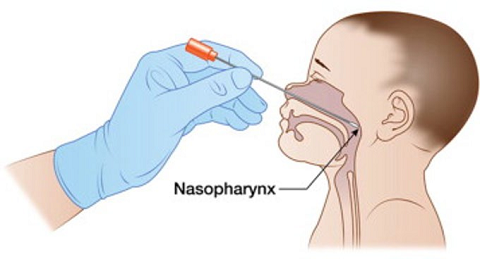
Figure 2: Collection technique for a nasopharyngeal swab
Sampling for the purposes of rapid antigen testing (RAT):
- The sampling requirements for COVID-19 RAT vary according to the specific kit in use
- Refer to the relevant kit insert for instructions on specimen collection and test performance
COVID-19 NAAT test reporting
Positive COVID-19 NAAT results
- The treating team does not need to make a Public Health notification for a positive SARS-CoV-2 PCR result as an automatic notification will be generated by the testing laboratory
- Families should be encouraged to register all positive COVID-19 RAT results online with WA Health
- If an inpatient, the treating team can register the positive COVID-19 RAT on the behalf of the patient
COVID-19 serological testing
- Serology is not currently used for diagnosis of acute illness but may have a role in retrospective confirmation of past SARS-CoV-2 infection.
- SARS-CoV-2 serology is recommended as part of the work-up for the Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS)
Other investigations in the setting of acute COVID-19 infection
- The role of laboratory and radiological investigations is in working through differential diagnoses (including viral co-infection and/or secondary bacterial infection) and in the detection of complications
- Investigations that may be indicated for positive COVID-19 cases dependent on clinical presentation include:
- Chest x-ray
- Bloods:
- Assessment of end organ function including blood gas/lactate, FBP, UEC, LFTs, coagulation profile
- Inflammatory markers including C-reactive protein (CRP)
- Sterile site cultures (blood, urine, etc.)
Infection control principles (Figures 3 & 4)
Transmission-based precautions
- Standard precautions apply to all patients at all times.
- In addition, contact and airborne precautions (Fit-tested PFR, protective eyewear, gown and gloves) are required for care of all patients who fulfil the positive COVID-19 case definition
- This also applies to asymptomatic close contacts and symptomatic patients in the hospital environment who are awaiting COVID-19 test results.
- All efforts should be made to reduce the need for AGPs. Where required, healthcare workers (HCWs) should use a fit-tested PFR, protective eyewear, gown and gloves as per standard transmission-based precautions.
- The CAHS Transmissible Diseases Index (TDI) should be referred to for information on required transmission based precautions where an alternate infectious disease diagnosis or clinical syndrome exists.
- If more than 4 weeks have passed since release from isolation, recovered cases who are symptomatic should be tested for SARS-CoV-2 and placed on contact and airborne precautions. If they test positive for SARS-CoV-2 they should be managed as a COVID-19 positive case or be managed as a close contact if they meet the close contact definition.
Bed allocation
For all positive COVID-19 patients and asymptomatic close contacts, ensure the following:
- Prioritise the patient for admission to a NPIR.
- Preference should be given to the following patient groups if NPIR capacity is limited:
- Positive COVID-19 cases
- Asymptomatic close contacts that require aerosol generating procedures
- A single room with door closed & portable HEPA filter is otherwise sufficient provided staff members use contact and airborne transmission-based precautions on entry to the room.
Refer to Figure 3 for further information on recommended bed allocation and transmission-based precautions for unplanned admissions dependent on COVID-19 status.
Inpatient de-escalation of isolation & transmission-based precautions
- Given that healthcare facilities remain high-risk settings, positive COVID-19 cases (confirmed or probable) should complete 7 days of isolation with contact and airborne precautions in place if inpatient care is required during this period.
- Children who require ongoing inpatient care following completion of 7 full days of isolation from the time of COVID-19 diagnosis should be discussed with Infection Prevention & Control (IP&C) prior to de-escalating precautions (Figure 5):
- Immunocompetent patients may be moved out of a NPIR provided there has been resolution of acute symptoms and they have a negative COVID-19 RAT
- Immunocompromised patients may need to remain under precautions for a longer period given the risk for ongoing viral shedding.
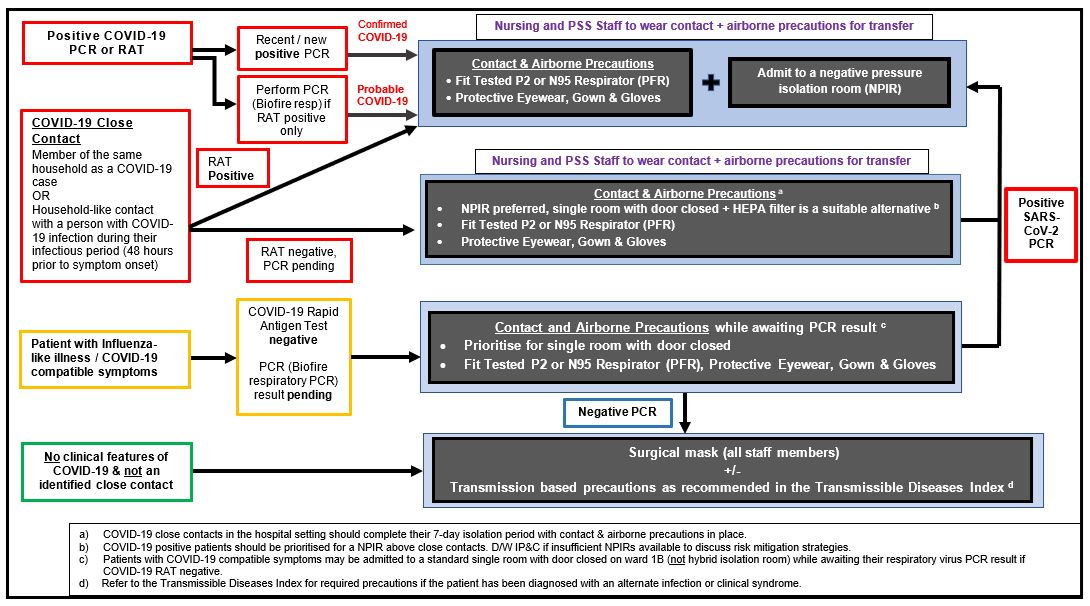
Figure 3: Recommended COVID-19 Testing & Transmission-based Precautions for Unplanned Admissions
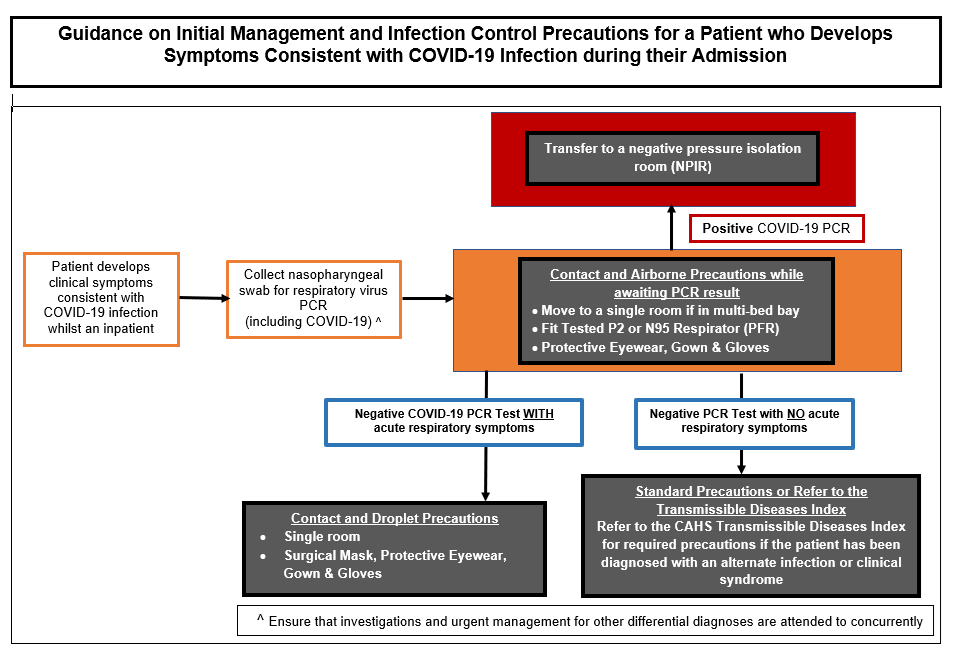
Figure 4: Initial Management & Infection Control Precautions for Inpatients who Develop COVID-19 Compatible Symptoms during their Admission
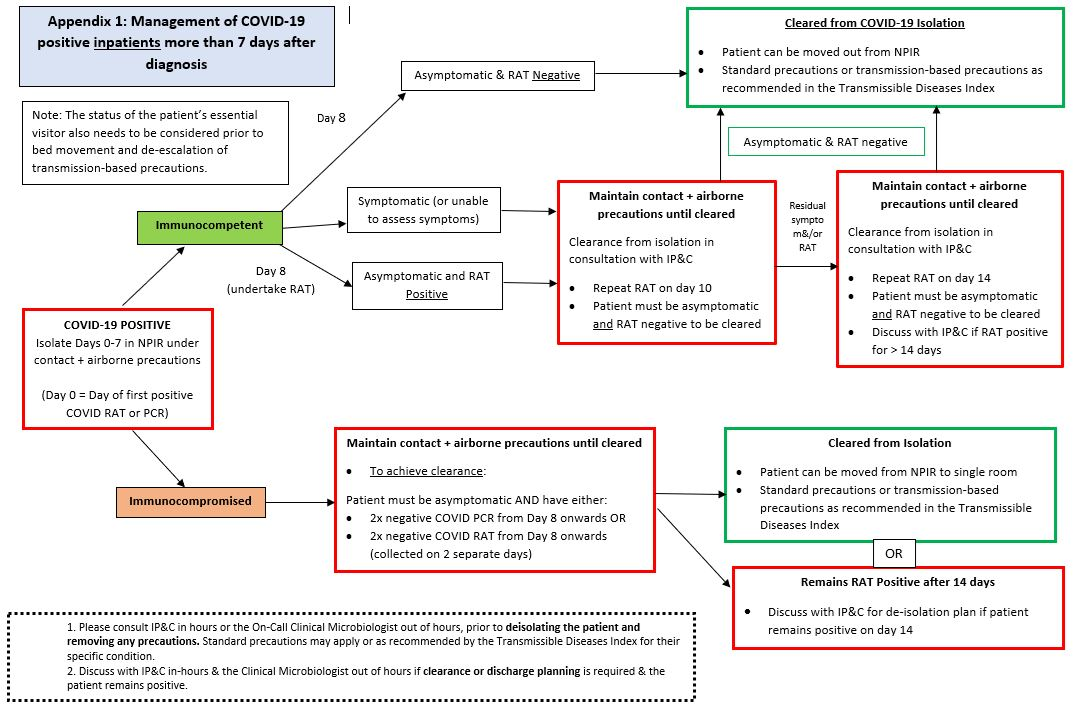
Figure 5: Recommended Transmission-based Precautions for COVID-19 Positive Inpatients following Completion of 7 days Isolation
Aerosol generating procedures (AGPs)
- AGPs are those that stimulate coughing and thereby promote the generation of fine respiratory particles. They may increase the risk of infection transmission via the airborne route. AGPs include but are not limited to:
- Tracheal intubation and extubation
- Intentional or inadvertent disconnection / reconnection of closed ventilator circuit
- Open oropharyngeal or nasopharyngeal suctioning
- Upper respiratory tract instrumentation and/or surgery (e.g. bronchoscopy, tracheotomy)
- Manual or non-invasive ventilation (including BiPAP and CPAP)
- Sputum induction & chest physiotherapy
- High flow nasal oxygen (HFNO)
- Cardiopulmonary resuscitation
- Nebuliser use
- The following are not considered AGPs: NGT insertion, nasopharyngeal swab collection, low flow oxygen therapy and Nitrous Oxide administration for procedural sedation.
- Senior medical input should determine if the AGP is warranted for a positive COVID-19 case based on clinical need. Decision making should be based on likely clinical benefit and whether an alternative exists.
- Nebulisers should be avoided and replaced by single patient use spacers where clinically appropriate.
- If an AGP is deemed essential, the following precautions should be undertaken:
|
Positive COVID-19 Case or Close Contact
|
No Clinical Features or Epidemiological Risk for COVID-19 Infection
|
|
Prioritise for a negative pressure isolation room (NPIR)
|
Move to a single room with door closed
|
|
Limit HCWs in the room to those who are essential
|
Limit HCWs in the room to those who are essential
|
|
Attending staff must wear PPE as for contact + airborne precautions (fit-tested PFR, protective eyewear, gown and gloves)
|
Attending staff must wear PPE as for contact + airborne precautions (fit-tested PFR, protective eyewear, gown and gloves)
|
- For detailed Infection Prevention & Control advice please refer to the following:
Staff allocation
Refer to the Employee Health Infections policy within the CAHS Infection Control Policy manual for further information.
Outpatients
- For outpatients, if the appointment cannot be postponed or performed via Telehealth for symptomatic patients, a COVID-19 RAT is suitable to guide the necessary infection control risk mitigation plan and transmission-based precautions for the duration of the patient visit.
- COVID-19 positive patients may be seen in Clinic C with contact & airborne precautions in place given prior arrangement.
- The day treatment unit has a functional NPIR which can be utilised for COVID-19 positive patients.
Management of positive COVID-19 cases
Management based on clinical manifestation
- Thresholds for admission/transfer of patients with acute respiratory illness currently should not change. These decisions should be based on the clinical severity of disease, not the presumed underlying viral aetiology.
- The management of suspected COVID-19 cases should follow existing PCH management guidelines including:
Management based on severity of illness
Refer to Appendix 1 for disease severity classification in children and adolescents.
Mild Illness
- Children with mild disease may not require admission to hospital if respiratory and hydration status are stable and social circumstances permit ongoing care and isolation at home.
- Symptomatic management is recommended.
- Families of COVID-19 positive children who are discharged from hospital should be advised that they need to complete the required isolation period at home following the diagnosis of COVID-19.
- Refer Department of Health advice on COVID-19 isolation for up to date information on isolation duration and requirements for positive COVID-19 cases and their close contacts.
Moderate Illness
- Children with moderate disease require admission to hospital for oxygen therapy and hydration.
- Refer to the Oxygen Administration Guideline within the PCH Clinical Practice Manual for details.
Severe Illness
- Children with severe illness should be admitted to hospital and discussed with both the admitting consultant and the Paediatric Critical Care Unit.
Respiratory Support
- Conventional oxygen therapy [nasal prong, mask or high-flow nasal oxygen (HFNO)] progressing to or non-invasive ventilation (NIV) should be used for neonates, children and adolescents where oxygen saturations cannot be maintained within the target range.
- As HFNO and NIV are AGPs, positive COVID-19 cases requiring this support should be discussed with a senior clinician prior to commencement and should be prioritised for admission to a NPIR under contact & airborne transmission-based precautions.
Critical Illness
- Increase inspired oxygen concentration as required.
- Consider endotracheal intubation and mechanical ventilation in neonates, children and adolescents with COVID-19 who continue to deteriorate despite optimised non-invasive respiratory support*.This should be discussed with a PCH PCC consultant.
- Endotracheal Tube (ETT) suction should be minimised but may be essential. It should be performed with in-line suction apparatus incorporated into the airway circuit at the time of intubation.
Disease modifying treatments
- Additional therapies may be considered for children at high risk for severe disease (e.g. Haemopoietic stem cell transplant recipient, solid organ transplant recipient, severe primary immunodeficiency) &/or those requiring hospital admission for respiratory support.
- The use of disease-modifying therapies for acute COVID-19 infection should be discussed with a Paediatric Infectious Diseases consultant. Treatments that may be considered due to ongoing demonstrated activity against more recent SARS-CoV-2 variants include:
- Dexamethasone (where supplemental oxygen is required)
- Remdesivir: National Medicine Stockpile approval required
- Paxlovid® (Nirmatrelvir/Ritonavir): National Medicine Stockpile approval required
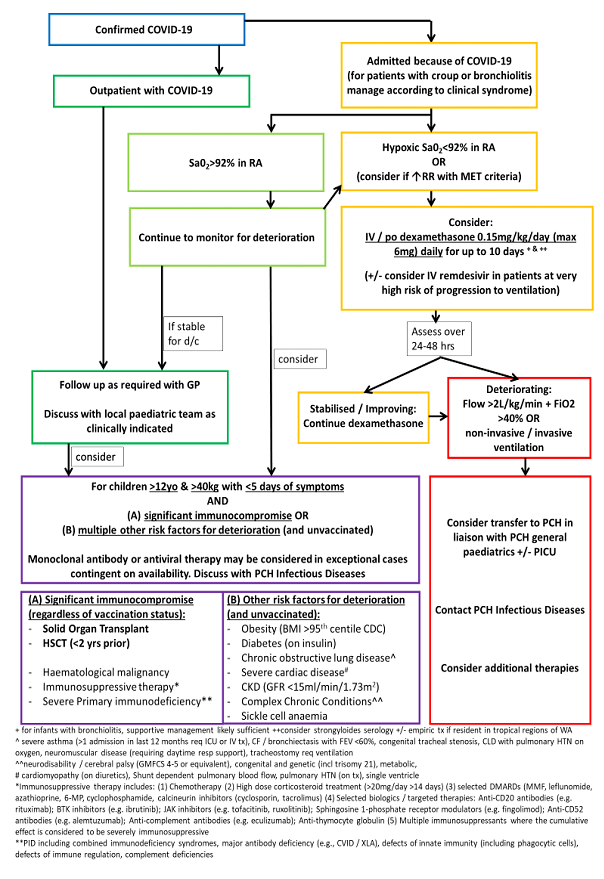
Figure 6: COVID-19 Patient Treatment
Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS)
Long COVID
- Most children and adolescents with COVID will recover uneventfully and be back to their usual health within 1-2 weeks. A smaller number will have some persistent symptoms for a few weeks until they are fully recovered. A much smaller number may have symptoms for up to 3 months but can also be expected to make a full recovery.
- Studies to date have found that children and adolescents are much less likely to have long COVID than adults and recover faster than adults. Long COVID is very rare in younger children and mainly affects children over 10 years.
Definition
Long COVID is a term used to describe individuals with physical symptoms that continue for more than 12 weeks after testing positive for SARS-CoV-2, that cannot be explained by an alternative diagnosis and which are having an impact on that person’s day-to-day functioning.
Symptoms
The most common symptoms include fatigue, headaches, difficulty concentrating (“brain fog”), changes to sense of taste and smell, muscle aches and joint pains, cough, breathlessness, chest pain, palpitations, dizzy spells and low mood.
Symptoms are very similar to those experienced by children and young people recovering from other viral infections, such as glandular fever.
Assessment
If a child or adolescent has symptoms persisting beyond 4 weeks after testing positive for SARS-CoV-2, they should be encouraged to see their GP for a detailed assessment, to exclude other causes.
The GP may consider further investigations depending on the symptoms and clinical signs. As per RACGP Caring for Patients with post-COVID-19 Conditions Guideline:
- History should include symptoms enquiry and the severity of their acute COVID infection, as well as vaccination status and dates. All adolescents should have a psychosocial screen (HEADSS) as part of their assessment
- Clinical assessment should include observations (heart rate, respiratory rate), pulse oximetry for oxygen saturation, blood pressure, weight, examination of the cardiovascular, respiratory and abdominal systems.
Investigations
Depending on the differential diagnosis, those to consider include:
1/ Bloods: FBC, iron studies, U&Es, LFTs, TFTs, CRP, glucose, coeliac serology, CK, B12/folate, vitamin D, troponin and D-dimers
2/ Diagnostic imaging: CXR, ultrasound scan
3/ Other: ECG, Holter monitor, ECHO, pulmonary function tests
Management
- There is no specific treatment for Long COVID.
- Refer to the RACGP Caring for Patients with post-COVID-19 Conditions Guideline for advice on management of common symptoms.
- Children/adolescents and their families should ensure that:
- Routines are in place & the child/adolescent is getting out of bed during the day;
- The child/adolescent is eating and drinking well;
- The child/adolescent is doing gentle graded exercises;
- The child/adolescent is keeping in touch with family and friends.
- Parents/carers should make contact with their child’s school if poor school attendance is occurring or anticipated. Support for school re-integration is an essential component of clinical management.
- Allied Health services (psychology, physiotherapy, occupational therapy) may be useful to support the child/adolescent through recovery.
- For children/adolescents who have had the preliminary work up with their GP and have symptoms persisting for more than 12 weeks and not improving, consider an outpatient referral to the local general paediatric service, or a PCH paediatric subspecialist depending on the symptoms and signs.
- The focus of the paediatric assessment will be to:
1/ exclude an alternative diagnosis that would explain the child’s symptoms
2/ support a graduated rehabilitation program
Discharge of positive COVID-19 patients from PCH
- Refer to the WA Health policy Coronavirus Disease - 2019: Infection Prevention and Control in Western Australian Healthcare Facilities for further advice on this subject.
- Where discharge to a residential facility is planned, the decision to do so must be agreed by a senior medical practitioner and the residential care facility, taking into account whether the facility is equipped to provide the ongoing clinical care of the patient.
- The following principles apply for transport of COVID-19 positive patients following discharge:
- A private vehicle should be the first choice of transport for discharging a patient to their home or accommodation (family’s car or a lift from a family member).
- The patient should wear a clean surgical mask provided by the hospital, where possible, unless the patient has already been cleared by time of discharge.
- All occupants of the vehicle, including the driver, should be encouraged to wear a surgical mask.
- If a private vehicle is not available, a taxi or rideshare may be used. The driver should be informed of the patient’s COVID-19 status.
- The patient (& family members) and driver are recommended to wear a surgical mask.
- Alternatively, the hospital’s patient transport service (PTS) can be used with their standard vehicle cleaning procedure after drop-off of the patient and family.
- Transfer of a COVID-19 positive patient between healthcare facilities should only occur if medically required.
- Patients should wear a surgical mask for the duration of the transfer
- If on oxygen therapy, the patient should be transitioned to nasal prongs if their conditional allows. If not, a surgical mask can be placed over the oxygen mask prior to transport.
Appendix 1: Definition of illness severity for children and adolescents
| |
Feeding/Hydration |
Respiratory / Vital Signs |
Oxygen requirement |
| Mild illness |
Normal or mildly reduced feeding |
No or mild upper respiratory tract symptoms OR
No or mild work of breathing |
No supplemental oxygen required |
Moderate illness
|
Unable to maintain hydration without NG or IV fluids
AND
Normal conscious state |
Moderate work of breathing OR
Abnormal vital signs for age but does not persistently breach early warning criteria OR
Brief, self-resolving apnoea (infants)
|
Requires low-flow oxygen
AND/OR
Chest x-ray changes due to COVID-19 without other severe features |
| Severe illness |
Unable to maintain hydration without NG or IV fluids
OR
Drowsy/tired but easily rousable |
Moderate-severe work of breathing OR
Abnormal vital signs for age with breaches of early warning criteria OR
Apnoea needing support / stimulation (infants) |
Requires high-flow oxygen or non-invasive ventilation |
| Critical illness |
Unable to maintain hydration without NG or IV fluids
OR
Altered conscious state / unconscious |
Unable to maintain breathing or prevent apnoea without advanced modes of support OR
Abnormal vital signs for age with persistent breaches of early warning criteria OR
Haemodynamically unstable without inotropic or vasopressor support OR
Other organ failure |
Requires advanced modes of support to maintain oxygenation:
Intubation and mechanical ventilation OR Extracorporeal membrane oxygenation (ECMO) |
| Based on the Australian Guidelines for the Clinical Care of People with COVID-19: Definition of disease severity for children and adolescents. National COVID-19 Clinical Evidence Taskforce |
References
- Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinical Medicine 2020 (July); 24:100433.
- Munro APS, Faust SN. COVID-19 in children: current evidence and key questions. Curr Opin Infect Dis 2020; 33(3): 540-547.
- Brewster DJ, Chrimes N, Do TBT, Fraser K, Groombridge CJ, Higgs A, et al. Consensus statement: safe airway society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust 2020; 212(10): 472-481.
| Approved by: |
CAHS COVID Executive Oversight Committee (CCEOC) |
Date: |
Dec 2022 |
| Endorsed by: |
CCEOC Chair |
Date: |
Dec 2022 |
This document can be made available in alternative formats on request for a person with a disability.