Poisoning - Paracetamol
Disclaimer
These guidelines have been produced to guide clinical decision making for the medical, nursing and allied health staff of Perth Children’s Hospital. They are not strict protocols, and they do not replace the judgement of a senior clinician. Clinical common-sense should be applied at all times. These clinical guidelines should never be relied on as a substitute for proper assessment with respect to the particular circumstances of each case and the needs of each patient. Clinicians should also consider the local skill level available and their local area policies before following any guideline.
Read the full CAHS clinical disclaimer.
|
Aim
To guide staff with the assessment and management of paracetamol poisoning in children.
This guideline provides an outline of the general approach to paracetamol poisoning. Specific information about poisoning presentations can be obtained from Poisons Information Service 13 11 26 or refer to Toxicology and Toxinology: Paracetamol Poisoning: advice for primary care providers – Therapeutic Guidelines (External website).1
Background
Paracetamol overdose can occur as either a single large ingestion, usually as a deliberate self-poisoning, or as an accidental supra-therapeutic ingestion over days.
- Morbidity and mortality is from hepatic injury. Life-threatening liver failure is rare.
- N-Acetyl Cysteine (acetylcysteine) is an effective and well-tolerated antidote and if given within 8 hours of ingestion, is almost 100% effective at preventing liver injury.
Limitations of this guideline
- This guideline will cover single overdoses, or staggered ingestions over a period of less than 8 hours of the immediate-release preparation of paracetamol.
- Supra-therapeutic overdoses of >60 mg/kg/day in children (>4 g/day in adults), or those involving modified release preparations (e.g. Panadol Osteo®, Duatrol SR®) cannot be interpreted using the nomogram, and advice should be sought from the Poisons Information Service 13 11 26 or local toxicology services.
Principles
Management - approach to paracetamol toxicity
Resuscitation
-
Resuscitation is only required in the event of co-ingestion of other agents, or in the rare event of massive acute ingestions causing coma, severe lactic acidosis and hypoglycaemia.
Risk assessment1
- The threshold for hepatic injury following an acute ingestion varies, but is generally around 200 mg/kg.
- Note, exposure in mg/kg should be based on Ideal Body Weight.
- The risk of hepatotoxicity (without treatment with acetylcysteine) can be determined by plotting the post-ingestion paracetamol level on the nomogram below.
- Time of ingestion:
- If time of ingestion is unknown, assume that it took place at the earliest possible time, to give the worst-case scenario.
- Patients presenting over 8 hours after ingestion with elevated transaminases are assumed to have early toxicity.
- Patients presenting more than 24 hours post ingestion with no elevated transaminases and a negative paracetamol level have a negligible risk of toxicity.
Clinical features
- Most patients will initially present with no symptoms, or only mild gastrointestinal symptoms.
- Massive overdose is rare, but may cause coma and metabolic acidosis.
Four chronological phases are described in cases of significant acute overdose:
- Under 24 hours
- Asymptomatic
- Mild nausea and vomiting
- 1 to 3 days
- Right upper quadrant tenderness
- Hepatotoxicity
- 3 to 4 days (severe cases)
- Fulminant hepatic failure and / or death
- Acidosis
- Renal failure
- Day 4 to 2 weeks
Supportive care and monitoring
- General supportive care, such as IV fluid, anti-emetics, thromboembolic prophylaxis should be given as required on a case by case assessment.
- Patients with rising transaminases or INR >2.5 should have four hourly observations and blood glucose (BGL) monitoring.
Investigations 2
- Screening tests in deliberate paracetamol overdoses should include an electrocardiogram (ECG) and BGL.
- When a patient has taken an overdose of another agent, screening paracetamol levels should be taken any time after 30 minutes post-ingestion.
- If paracetamol is detected, a 4 hour level (post-ingestion) should be taken.
Table 1: Acute ingestion of immediate release paracetamol
< 8 hours
|
8-24 hours |
> 24 hours |
| Serum paracetamol level |
At 4 hours post ingestion or as soon after as possible
|
At presentation |
At presentation |
Transaminases (ALT, AST)
|
With initial paracetamol level and at 18 hours post commencement of the 2 bag acetylcysteine infusion treatment, before stopping the acetylcysteine infusion.
|
At presentation and at 18 hours post commencement of the 2 bag acetylcysteine infusion treatment, before stopping the acetylcysteine infusion. |
At presentation and at 18 hours post commencement of the 2 bag acetylcysteine infusion treatment, before stopping the acetylcysteine infusion. |
| INR
|
Not indicated
|
Not indicated
|
At presentation
|
| Creatinine and urea |
Not indicated |
Not indicated |
At presentation
|
| Blood glucose |
Not indicated |
Not indicated
|
At presentation
|
| Blood gas |
Not indicated
|
Not indicated
|
At presentation |
Liquid paracetamol ingestion (< 6 year olds)4
- In asymptomatic children < 6 years of age who have ingested ≥ 200 mg/kg of liquid paracetamol, a 2 to 4 hour level can be used instead of a 4 hour level.
- If the 2 to 4 hour level is < 150 mg/L, a 4 hour level is not required and the patient is cleared.
- If the 2 to 4 hour level is ≥ 150 mg/L, a 4 hour level should be measured.
- If the 4 hour level is ≥ 150 mg/L acetylcysteine therapy is indicated4.
Staggered ingestions within < 8 hour period6
- Staggered ingestions (in the setting of deliberate self-poisoning) should be managed as an acute immediate release ingestion assuming the total dose was taken at the earliest possible time.
- If the level is taken < 2 hours since the last ingestion, it should be repeated at 4 hours after the last ingestion and plotted on the nomogram using the earliest ingestion as time of ingestion.
- If > 8 hours since the first ingestion, acetylcysteine should be started on arrival.
Decontamination - activated charcoal
- Activated charcoal is never indicated in children less than 6 years old with isolated paracetamol overdose or any liquid paracetamol ingestion.
- A single dose of 1 g/kg (up to 50 g) should be given to an awake, co-operative, older child if they present within 2 hours of ingestion AND the calculated dose is toxic (>10 g or ≥ 200 mg/kg)3
- If > 30 g or > 500 mg/kg of paracetamol has been ingested, this dose should be offered up to 4 hours post ingestion.
Enhanced elimination
Antidote - Acetylcysteine
- Intravenous acetylcysteine is indicated in all patients who have a risk assessment suggesting the potential for hepatotoxicity or who present with clinical symptoms of hepatic injury.
- Acetylcysteine standard dose regimen is a 2 bag infusion given over 20 hours.
- Management and use of acetylcysteine in patients with a known time of overdose is shown in the flow chart below.
Figure 1: Treatment nomogram
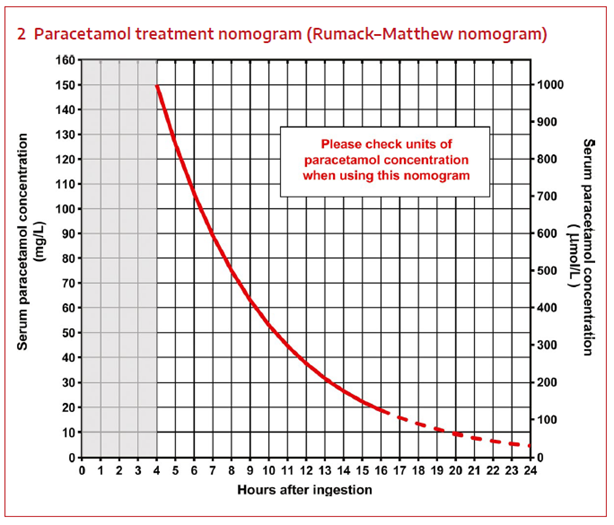
*Ensure that correct units are used (i.e. µmol/L or mg/L)
At PCH, paracetamol levels are measured as mg/L which is read on the left side of graph.
*Adapted from Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand.4
Figure 2: Acute immediate release paracetamol ingestion management2
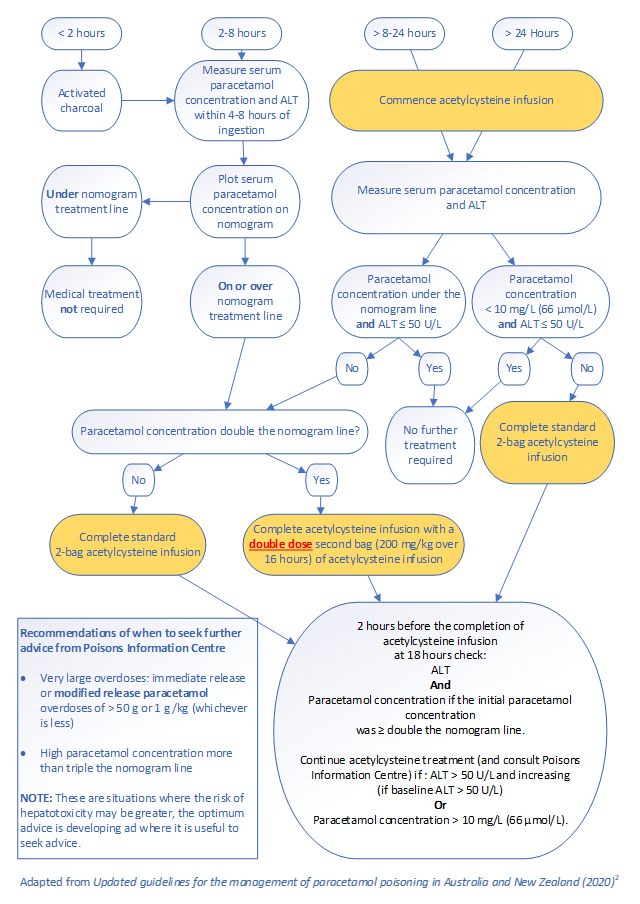
- Patients who present > 8 hours after ingestion OR with an unknown time of ingestion must have an acetylcysteine infusion commenced on arrival. The acetylcysteine may be ceased when laboratory testing confirms a non-toxic overdose or on advice from toxicology services.
- If the paracetamol level is greater than or equal to double the nomogram line, the dose of acetylcysteine in the second bag should be doubled.
- In such cases typically, > 30 g or > 500 mg/kg is ingested. These massive ingestions should be discussed with Toxicology.
- All patients receiving acetylcysteine need an ALT level taken at 18 hours following commencement i.e., 2 hours before the end of the second (16 hour) infusion. A paracetamol level should also be taken if the initial level was greater than or equal to double the nomogram line.
- Continuation of the infusion beyond 20 hours may be indicated in patients with delayed presentations, repeated supra-therapeutic ingestions, massive ingestions, or those with an increase in ALT.
- The second infusion dose should be repeated until transaminase levels are falling, there is no detectable paracetamol and the patient is clinically improving.
- Acetylcysteine is known to cause an immune mediated reaction in 10-50% of patients, with symptoms including bronchospasm, hypotension, flushing, rash and angio-oedema.
- Reactions typically occur after the first bag and can be treated using an oral antihistamine such as loratadine or promethazine and salbutamol.
- Cessation of the infusion should only occur in severe reactions and should be restarted once the reaction is settling. This reaction does not indicate anaphylaxis.
- Patients should be cardiac monitored for the first infusion bag, monitoring can be discontinued after this time unless an anaphylactic reaction has occurred, or otherwise clinically indicated.
- The dosage of acetylcysteine in children is the same as in adults but should be infused in smaller volumes of glucose 5%.
Acetylcysteine infusion protocol in acute paracetamol overdose
Intravenous acetylcysteine for paracetamol poisoning2
- Refer to PCH Medication Monograph Acetylcysteine (Medication Management Manual, internal WA Health only)
- Use actual measured body weight to calculate acetylcysteine doses (use ideal body weight to quantify extent of paracetamol overdose in obese children)3
- Smaller volume for dilution may be used in younger infants or fluid restricted patients (7 mL/kg for the first infusion, 14 mL/kg for the second infusion).
- Alternative diluents other than glucose 5% can be used if clinically necessary (e.g sodium chloride 0.9%, or glucose 5% with sodium chloride 0.9%, if hyponatraemia is of concern).3
- The dose of acetylcysteine in the second infusion bag should be doubled (200 mg/kg maximum 22 g) if the paracetamol level is greater than or equal to double the nomogram line.
- If ongoing acetylcysteine infusion is required, repeat second infusion.
- Refer to PCH Medication Monograph Acetylcysteine (PCH Medication Monograph - internal WA Health only).
NOTE: Both infusions should be administered: dosing regimen takes 20 hours.
Infants or children < 20kg
| First infusion* |
200 mg/kg |
100 mL |
4 hours |
| Second infusion* |
100 mg/kg |
250 mL |
16 hours |
(*always prescribe both infusions)
Children 20 - 50kg
| First infusion* |
200 mg/kg |
250 mL |
4 hours |
| Second infusion* |
100 mg/kg |
500 mL |
16 hours |
(*always prescribe both infusions)
Children or adolescents > 50kg
(Recommended ceiling weight: 110kg)
| First infusion* |
200 mg/kg |
500 mL |
4 hours |
| Second infusion* |
100 mg/kg |
1000 mL |
16 hours |
(*always prescribe both infusions)
Disposition and follow-up
- Medical discharge can be given in the following scenarios:
- Patients with a 4 hour paracetamol level below 150 mg/L.
- Patients presenting within 8 hours after ingestion with a level below the nomogram line.
- Patients presenting between 8-24 hours after ingestion with a level below the nomogram line and a normal ALT.
- Patients presenting > 24 hours after ingestion with an undetectable paracetamol level and a normal ALT.
- All patients who have received acetylcysteine (i.e., the full 20-hour regimen) within 8 hours of ingestion (except for those with massive overdoses) with a normal (18 hour) ALT at the end of the infusion.
- Patients who have received acetylcysteine (i.e., the full 20-hour regimen) whose transaminases are improving, are clinically well, and have an undetectable paracetamol level (if initial level was greater than or equal to double the nomogram line)
- All cases of deliberate poisoning should have a psychiatric assessment.
When to seek additional advice
- Overdose of modified release preparations
- Repeated supra-therapeutic overdoses
- Massive overdoses (> 500 mg/kg)
- Delayed presentations with elevated transaminases.
Nursing
Observations
- Complete and record a full set of observations on the Observation and Response Tool and record additional information on the Clinical Comments chart.
- Complete a full set of neurological observations.
- Patients requiring IV acetylcysteine
- Measure heart rate, respiratory rate, blood pressure, oxygen saturation every 30 minutes for the first 2 hours then hourly if stable.
- Observe for localised reaction and anaphylactic reactions as per above medical guidelines.
- Minimum of hourly visual observations should be recorded whilst in the emergency department, with additional observations as clinically indicated.
- Any signs of clinical deterioration should be reported immediately to the medical team.
- Apply a topical local anaesthetic cream approximately 1 hour prior to expected time of blood collection. e.g., lidocaine 2.5% with prilocaine 2.5% cream or lidocaine 4% cream.
References
- Toxicology and Toxinology: Paracetamol Poisoning: advice for primary care providers – Therapeutic Guidelines Cited July 2022. Last Updated: August 2020.
- Chiew A, Reith D, Pomerleu A, Wong A, Isosardi KZ, J Soderstrom, Buckley N. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust 2019; p1-9. doi: 10.5694/mja2.50428
- Toxicology Handbook, 4th Edition 2022. Armstrong J, Pascu O. Elsevier Australia
(available via ClinicalKey under Useful Resources section above)
- Micromedex® 2.0. Greenwood Village, Colorado, USA: Truven Health Analytics; 2019 [Available from: http://www-micromedexsolutions-com.pklibresources.health.wa.gov.au/.
- Paracetamol Poisoning [published August 2020]. In: eTG complete [digital]. Melbourne: Therapeutic Guidelines Limited, 2021 Feb, available from: https://tgldcdp-tg-org-au.pklibresources.health.wa.gov.au/viewTopic?topicfile=toxicology-paracetamol-liquid-in-child-under-six
| Endorsed by: |
Drugs and Therapeutics Committee |
Date: |
May 2024 |
This document can be made available in alternative formats on request for people with disability.